Onkopedia Guidelines Polycythaemia Vera (PV) 2023
Polycythaemia Vera (PV) | Changing therapy
A lot has changed in recent years, both in the diagnostic criteria and in the treatment of PV. The disease PV can now be diagnosed even more precisely and also treated more individually. This article will highlight the most important points of the current Onkopedia Polycythaemia Vera guideline.
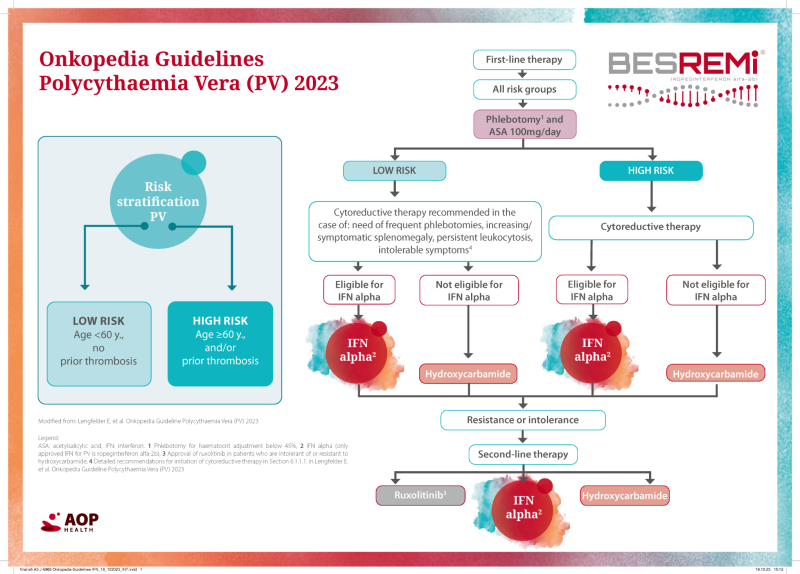
Relevant changes compared to the previous version
(Onkopedia Guidelines PV 2023 vs. 2021)
• Changed treatment algorithm: Recommendation of first-line use interferon alpha for all IFN-eligible patients.
Newly included data:
• Recommendation for the possible use of cytoreductive therapy in low-risk PV.¹
• Reduction of allele burden under therapy clinically relevant (EFS, PFS, OS).²
• Ropeg-IFN is highly efficient in reducing JAK2 allele burden.³
• Advantages of Ropeg-IFN over phlebotomy+ASA in terms of control of Hct <45% and disease progression.⁴
Link to Onkopedia
Literature
1) Marchetti M et al. 2022. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022 Apr;9(4):e301-e311. doi: 10.1016/S2352-3026(22)00046-1
2) Harrison CN et al. 2023. Ruxolitinib Versus Best Available Therapy for Polycythemia Vera Intolerant or Resistant to Hydroxycarbamide in a Randomized Trial. J Clin Oncol. 2023 Jul 1;41(19):3534-3544. doi: 10.1200/JCO.22.01935
3) Gisslinger H et al. 2022. Ropeginterferon alfa-2b achieves patient-specific treatment goals in polycythemia vera: final results from the PROUD-PV/CONTINUATION-PV studies HemaSphere 6():p 97-98, June 2022. | DOI: 10.1097/01.HS9.0000843676.80508.b5
4) Barbui T et al. 2023. Ropeginterferon versus Standard Therapy for Low-Risk Patients with Polycythemia Vera. NEJM Evidence 2023 May 15; 2(6); DOI: 10.1056/EVIDoa2200335